45 e liquid label requirements
Trendy E-Liquid Labels for Every Flavor If the e-liquid bottle label itself is too small to fit the warning, it must be sold in a container, wrapper, or carton that does include the warning. E-Liquid Label Design Elements. Now that we've discussed the requirements, it's time to dig into the key label design elements that make up a premium e-liquid bottle… › cosmetics › cosmetics-labelingSummary of Cosmetics Labeling Requirements | FDA Mar 17, 2022 · Liquid oral hygiene products (e.g., mouthwashes, fresheners) and all cosmetic vaginal products (e.g., douches, tablets) must be packaged in tamper-resistant packages when sold at retail.
eCFR :: 49 CFR Part 173 -- Shippers - General Requirements for ... 30/05/2021 · The Code of Federal Regulations (CFR) is the official legal print publication containing the codification of the general and permanent rules published in the Federal Register by the departments and agencies of the Federal Government. The Electronic Code of Federal Regulations (eCFR) is a continuously updated online version of the CFR. It is not an official …

E liquid label requirements
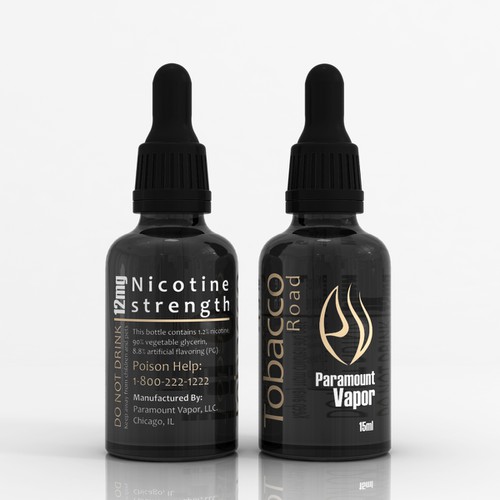
Deadline Approaching for Compliance with FDA's New E-Cigarette and E ... E-cigarettes and e-liquids must now have a warning label. The U.S. Food and Drug Administration (FDA) now requires a label on tobacco products warning that nicotine is addictive, beginning August 10, 2018, with a 30-day grace period. The requirement reflects the FDA's concern about rising e-cigarette use among young people. Best E Liquid UK - Vape Juice | Cheap E Liquid UK Browse our fantastic choice of cheap 100ml E liquid. Prices are as low as 0.35p per 10ml at Cheap e liquid UK with fast delivery. ... Our range includes a variety of flavours with best e liquid uk and we are wholesale bulk distributors of white label vapes. Besides, our lab-tested and pharmaceutical-grade, and we offer nicotine-free e-liquids ... Summary of Cosmetics Labeling Requirements | FDA 17/03/2022 · Liquid oral hygiene products (e.g., mouthwashes, fresheners) and all cosmetic vaginal products (e.g., douches, tablets) must be packaged in tamper-resistant packages when sold at retail.
E liquid label requirements. 1910.178 - Occupational Safety and Health Administration All new powered industrial trucks acquired and used by an employer shall meet the design and construction requirements for powered industrial trucks established in the “American National Standard for Powered Industrial Trucks, Part II, ANSI B56.1-1969”, which is incorporated by reference as specified in § 1910.6, except for vehicles intended primarily for earth moving or … E-cigarettes: regulations for consumer products - GOV.UK The requirements: restrict e-cigarette tanks to a capacity of no more than 2ml. restrict the maximum volume of nicotine-containing e-liquid for sale in one refill container to 10ml. restrict e ... Chemical Container Labels | EHS - University of Washington Hazard statement(s) (e.g., "toxic if inhaled" or "combustible liquid") GHS pictogram(s) ... Read the Hazard Communication Standard for Labels and Pictograms for more information about original container label requirements. Contact EH&S at 206.543.7388 or ehsdept@uw.edu for assistance in preparing a replacement label. Secondary chemical ... › articles › top-suppliersTop Private Label Soap Manufacturers, Suppliers, and Companies 1 day ago · Future International Diversified, Inc. makes a variety of different private label soaps (beauty, baby, shower gels, shampoos, antibacterial liquid soaps, etc.) in various shapes, styles, and sizes, and a choice of packaging. Minimum production starts at 50,000 soap bars, or 10,000 liquid soap units.
E Liquid Labels - Blank or Custom Printed | OnlineLabels® Represent your brand and personality with visually appealing e-liquid bottle labels. Create professional labels for your e-liquid, e-juice, e-cig, and vapor products with these perfectly sized blank or custom printed labels. This image features OL1000. No Minimum Orders. Lowest Price Guarantee. Does Your E-Liquid Packaging Meet Legal Requirements? 9 June 2021. E-Liquid packaging requires frequent changes to meet the proper legislation guidelines for trading. However, a recent study in Europe found that 86% of vaping products in fact, did not comply with the right packaging and labelling regulations. In the study, researchers took 49 products and found that in 42 of these, the labels were ... Labeling and Warning Statements for Tobacco Products | FDA 1. The United States District Court for the District of Columbia recently issued an order vacating the health warning requirements for cigars and pipe tobacco set forth in 21 CFR §§ 1143.3 and ... E-Liquid Vape Labels - Advanced Labels NW Quality e-liquid bottle labels make all the difference. Advanced Labels NW is a leader in label printing for vaping products, offering a wide range of custom e-liquid bottle labels and stickers to help you stay competetive in a growing market. We have worked with local and nationwide vape enthusiasts to bring their flavors to market with ...
Fda e liquid label requirements Jobs, Employment | Freelancer Search for jobs related to Fda e liquid label requirements or hire on the world's largest freelancing marketplace with 20m+ jobs. It's free to sign up and bid on jobs. › laws-regs › regulations1910.178 - Powered industrial trucks. | Occupational Safety ... All new powered industrial trucks acquired and used by an employer shall meet the design and construction requirements for powered industrial trucks established in the “American National Standard for Powered Industrial Trucks, Part II, ANSI B56.1-1969”, which is incorporated by reference as specified in § 1910.6, except for vehicles intended primarily for earth moving or over-the-road ... file.cop.ufl.edu › ce › consultwbCHAPTER 20 LABELING MEDICATIONS AND EXPIRATION DATING Label on and off sterile field NPSG.03.04.01: Label all medications, medication containers, and other solutions on and off the sterile field in perioperative and other procedural settings a. Follow standard labeling format above and label b. If not immediately administered, c. When transferred from original package to another container, d. › current › title-49eCFR :: 49 CFR Part 173 -- Shippers - General Requirements ... May 30, 2021 · (ii) Each salvage cylinder that is successfully requalified must be durably and legibly marked with the word “Tested” followed by the requalification date (month/year), e.g., “Tested 9/04.” The marking must be in letters and numbers at least 12 mm (0.5 inches) high.
E-Cigarette and E-Liquid Regulations in the European Union: An Overview Labeling requirements. E-liquid tanks and packaging shall carry a general warning, information message, health warning, and the children's resistance warning. Risks. Notice that some e-liquids produced outside the EU may contain harmful and restricted substances. Do not assume that all suppliers exporting e-liquids to the EU are selling ...
United Foods International, Inc. | Private Label Food Manufacturer We also offer hand assembly, shrink wrapping services, and custom label manufacturing made to your specifications and will be reviewed and approved to meet all requirements. Dry and Liquid Processing We have dry and liquid processing facilities well as an in-house research and development team and quality control personnel to assist you on any ...
Guide to E-Liquid (E-Juice) Labeling - Star Label Products E-Liquid Labeling Requirements E-liquid products are considered electronic nicotine delivery systems (ENDS), a form of tobacco product. The FDA requires any tobacco product intended for the manufacture, labeling, packaging, sale, advertising, promotion, import, and distribution in the United States to be properly labeled.
How to Label E-Liquid Bottles - OnlineLabels E-liquid bottles tend to come in standard sizes: 1 dram, 5 ml, 10 ml, 15 ml, 0.5 oz, and 30 ml. To accurately measure your bottle, download our printable ruler, cut it out, and wrap it around your bottle. Be sure to turn off the Page Scaling and Rotate and Center options to ensure it prints at actual size. Once you've got your measurements ...
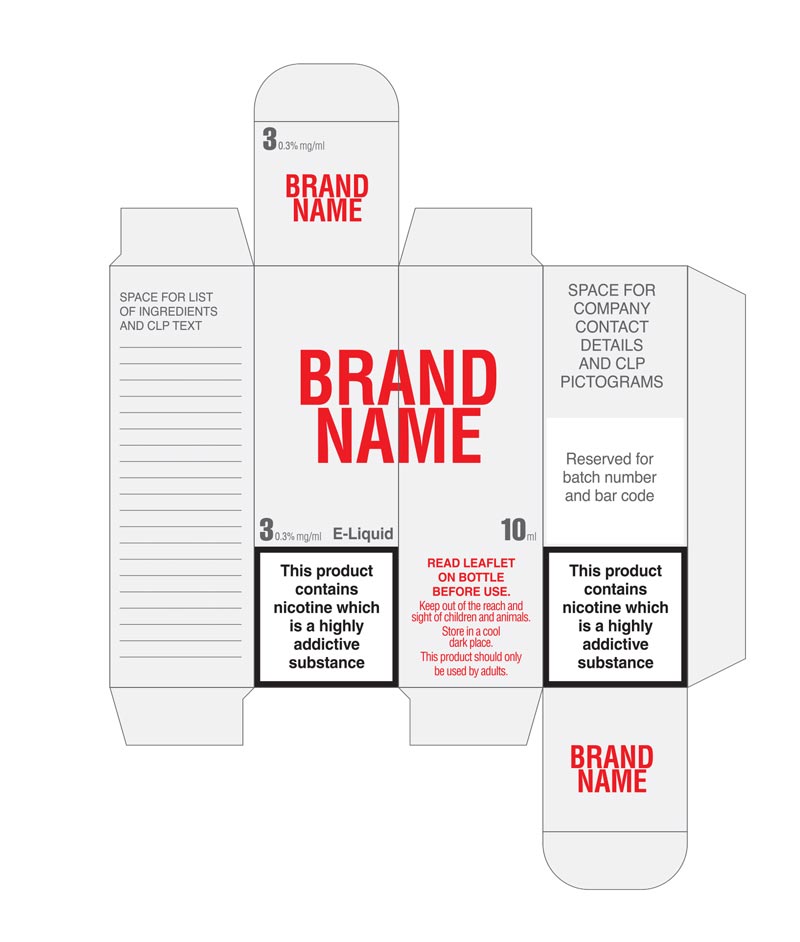
FDA E-Liquid Label Requirements - Custom Boxes with logo by Brilliant ... This required warning statement must also meet certain requirements, with respect to font, text, size, placement, and formatting of the FDA E-Liquid Boxes Label Requirements on the package labels. That is, the required warning statement on package labels must also appear directly on the package, and be clearly visible underneath any cellophane or other clear wrappings, per 21 CFR § 1143.3(a ...
Does your E-liquid label meet UK requirements? - Readability As you'll know by now, to sell an e-liquid product within the UK it must meet UK requirements, and it must be TPD compliant according to the EU directive. The information required to satisfy the TPD requirements is extensive. Readability's customers usually use a peel and reseal label or a single ply label with a printed carton (box) and a ...
TPD compliant e-liquid packaging. Part 1. - CHEMNOVATIC Hi Eric, We have designed the labels for VAPY e-liquids with the TPD in mind, hence they contain all the information required by the Directive. As for the exterior container , the TPD does not require e-liquids to have a box, the term "individual packaging" may as well refer to the bottle itself, nevertheless it still needs to display all the required information on the label, if the ...
E-Cigarettes, Vapes, and other Electronic Nicotine Delivery Systems ... ENDS may be manufactured to look like conventional combusted cigarettes, cigars, or pipes. Some resemble pens or USB flash drives. Larger devices, such as tank systems or mods, bear little or no ...
consolidatedlabel.com › label-articles › labelLabel Adhesives Guide: Types and Properties | Label Printing Feb 05, 2015 · Initial Tack: The immediate holding power of the label adhesive on contact with a specific surface. If initial tack is low, it will have low adhesion, allowing the label to be removed cleanly. Adhesives with a low initial tack will build up adhesion over time (i.e. the difference between removable and repositionable).
e liquid label requirements - YouTube e liquid label requirements
E-Liquid Warning Label Requirements for Sellers - Anthony Jones What are the e-liquid warning label TPD requirements? There are a number of requirements that e-cigarette and e-liquid labelling must now meet, including: Ingredients - all ingredients which make up 0.1% or more of the final formulation of the e-liquid must be listed. This must include the nicotine content. Health warning - labelling must ...
Interpretation of and Compliance Policy for Certain Label Requirement ... mix or prepare e-liquids or create or modify aerosolizing apparatus, also referred to as e- cigarettes, for sale or distribution (81 FR 28973 at 29046). B. Section 910 of the FD&C Act
Label Adhesives Guide: Types and Properties | Label Printing 05/02/2015 · Label Adhesive Guide Part 2: Performance and Selection. Now that you have a framework for how label adhesives work, the next part of our guide covers performance factors.Adhesive testing on the container you have chosen is extremely important for more complex labeling jobs to ensure you don’t end up wasting time and resources on labels that …
Labeling E-Liquids For E-Cigarettes - Griffin Rutgers With the tremendous growth in the vape and e-cig market, the FDA is cracking down on the requirements for what must be listed on the e-liquid labels. Packaging and labeling e-liquids for e-cigarettes is demanding and regulated. Whether you are a small 'mom & pop' producer, or a large company that mass produces of e-liquids, you must meet ...
› chemical-container-labelsChemical Container Labels | EHS - University of Washington To resize any of the Word or PDF label templates above to fit smaller containers, do the following: If the label template is a Word doc, fill in with the desired information, save it as a PDF, take a "snapshot" of the label in edit mode, paste it into a blank Word doc (this is now a picture), and resize at any corner of the image to desired size.
Summary of Cosmetics Labeling Requirements | FDA 17/03/2022 · Liquid oral hygiene products (e.g., mouthwashes, fresheners) and all cosmetic vaginal products (e.g., douches, tablets) must be packaged in tamper-resistant packages when sold at retail.
Best E Liquid UK - Vape Juice | Cheap E Liquid UK Browse our fantastic choice of cheap 100ml E liquid. Prices are as low as 0.35p per 10ml at Cheap e liquid UK with fast delivery. ... Our range includes a variety of flavours with best e liquid uk and we are wholesale bulk distributors of white label vapes. Besides, our lab-tested and pharmaceutical-grade, and we offer nicotine-free e-liquids ...
Deadline Approaching for Compliance with FDA's New E-Cigarette and E ... E-cigarettes and e-liquids must now have a warning label. The U.S. Food and Drug Administration (FDA) now requires a label on tobacco products warning that nicotine is addictive, beginning August 10, 2018, with a 30-day grace period. The requirement reflects the FDA's concern about rising e-cigarette use among young people.







































Komentar
Posting Komentar