43 which of the following is not an fda required component of a food packaging label?
What Is Required On A Food Label? - Chicago Tag Allergens: If a product contains any of the top eight allergens (milk, eggs, fish, shellfish, tree nuts, peanuts, wheat and soybeans) or is processed a facility that also processes those allergens, that must be disclosed on the label. Regulated Lingo which of the following is not required on a food label? (points : 2 ... The information on a food label. According to the food and drug administration (FDA), some of the information that are required on a food label include the following: Total calories; Total fat calories; Protein percentage; Name and address of the vendor. In conclusion, vitamin D percentage is an information which is not required on a food label.
CFR - Code of Federal Regulations Title 21 - Food and Drug … Verkko20.7.2022 · (a) Act means the Federal Food, Drug, and Cosmetic Act (sections 201-901, 52 Stat. 1040 et seq., as amended (21 U.S.C. 301-392)). (b) A custom device means a device within the meaning of section 520(b) of the Federal Food, Drug, and Cosmetic Act. (c) FDA means the Food and Drug Administration. (d) Implant means a device that is …
Which of the following is not an fda required component of a food packaging label?
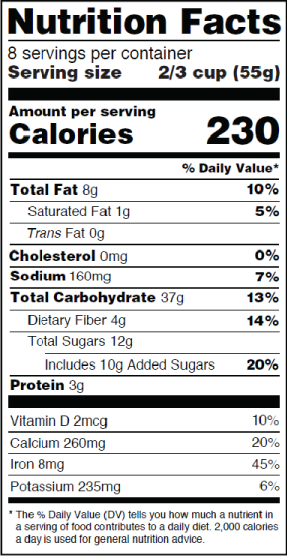
FDA Food Product Labeling & Packaging Requirements - ESHA Servings per container Mandatory nutrients (total calories, total fat, saturated fat, trans fat, cholesterol, sodium, total carbohydrate, dietary fiber, total sugars, added sugars, protein, vitamin D, calcium, iron, potassium) In a parenthetical directly after the name of the ingredient within the ingredient statement. Optional Nutrients On The Food Label - LabelCalc So if you are struggling with your nutrition facts label or just want to streamline the label creation process, an easy-to-use online label creation software that clearly denotes optional nutrients makes the task of creating your food label a breeze. LabelCalc offers FDA-compliant web-based nutritional analysis and label-making that is easy to ... Nutrition Labels 101: What's Required? What's Optional? The FDA requires nutrients that fall into one of these categories be listed on a nutrition label only when it's necessary to bolster or prove the label's food label or marketing claim. To best help consumers make informed decisions about their food choices, the FDA says all nutrition labels must include these 13 components.
Which of the following is not an fda required component of a food packaging label?. Guidance for Industry: Questions and Answers Regarding Food Allergens ... VerkkoFor questions regarding this document, contact Carol D'lima at the Center for Food Safety and Applied Nutrition (CFSAN) at 301-436-2371 (Updated phone: 240-402-1697) or Carol.Dlima@fda.hhs.gov ... CFR - Code of Federal Regulations Title 21 - Food and Drug Administration Sec. 211.180 General requirements. (a) Any production, control, or distribution record that is required to be maintained in compliance with this part and is specifically associated with a batch of a drug product shall be retained for at least 1 year after the expiration date of the batch or, in the case of certain OTC drug products lacking ... CFR - Code of Federal Regulations Title 21 - Food and Drug … Verkko20.7.2022 · (b)(1) When there is a deviation or unanticipated occurrence during the production and in-process control system that results in or could lead to adulteration of a component, dietary supplement, or packaging, or could lead to the use of a label not specified in the master manufacturing record, quality control personnel must reject the … Packaging, Labeling, Transporting, Storing — Food Law Label Approval FDA does not pre-approve labels; FDA may offer suggestions if the processor inquires; FDA will enforce law after the label is put in use. No pre-approval of label is required by FDA for products under its jurisdiction. It is the responsibility of the manufacturer or importer of a food to comply with current food labeling ...
5 Basic Elements that MUST be on Your Food Label Here are the five elements that must go on every food label according to the FDA: #1: Statement of Identity Your product must be clearly identified on the package label. For example, a sauce jar label could have something like "Ethiopian Sauce. Made with 35 herbs and spices!" You must also indicate the intended use. Must a packaging manufacturer be registered with the FDA? As defined in 21 CFR 1.225, domestic and foreign facilities that manufacture, process, pack, or hold food for human or animal consumption in the U.S. must register with the FDA. Please be advised that if you choose to proceed with registering, you must comply with all registration requirements and other statutory requirements of the FD&C Act ... Iron - Health Professional Fact Sheet FDA currently requires that iron-containing dietary supplements sold in solid form (e.g., tablets or capsules but not powders) carry the following label statement: “WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. CFR - Code of Federal Regulations Title 21 - Food and Drug Administration Sec. 211.122 Materials examination and usage criteria. (a) There shall be written procedures describing in sufficient detail the receipt, identification, storage, handling, sampling, examination, and/or testing of labeling and packaging materials; such written procedures shall be followed. Labeling and packaging materials shall be ...
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration you must clearly identify, hold, and control under a quarantine system for appropriate disposition any component, packaging, and label, and any product that you receive for packaging or... Guidance for Industry: Preparation of Premarket Submissions for Food ... VerkkoA FCS is any substance that is intended for use as a component of materials used in manufacturing, packing, packaging, transporting, or holding food if the use is not intended to have any ... Questions and Answers on Health Claims in Food Labeling | FDA The Nutrition Labeling and Education Act of 1990 (NLEA) directed FDA to issue regulations providing for the use of health claims. All health claims must undergo review by the FDA through a... PDF SUMMARY OF 5 REQUIRED FOOD LABEL COMPONENTS Label Layout Instructions ... the PDP. Positioning and type size for each component is tightly regulated. In addition, all IP components must be placed together without intervening material, starting at the top left of the panel. PDP 1. Product Identity 21 CFR 101.3 Must include the standard food name (for a standardized food) or a descriptive name (for a non-
MasteringNutrition 2d Flashcards | Quizlet which of following is an FDA required component of a food packaging label? food and drug administration (FDA) the agency that regulates the information on a food label is the... zero trans fat the FDA put seventeen companies on notice for printing misleading claims on food packaging. what type of claim did they take issue with? meat and poultry
Regulatory Status of Components of a Food Contact Material Components of a food packaging material used in compliance with a regulation in 21 CFR (174-179) need no further FDA review. Most of the regulated indirect food additives can be found in...
FDA Regulation of Adhesives in Food Packaging Hence, manufacturers of finished food packaging containing an adhesive meet the requirements of Section 175.105 only if the adhesive is used in a manner that prevents it from becoming a component of food or is otherwise used in a manner that does not result in its migration to food.
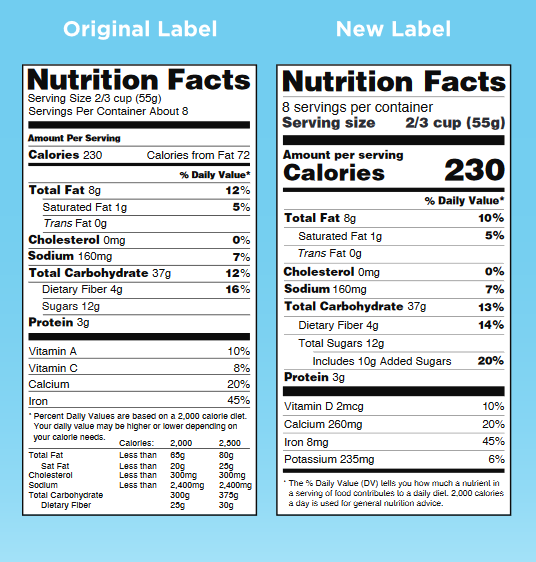
Changes to the Nutrition Facts Label | FDA - U.S. Food and Drug ... Verkko7.3.2022 · Manufacturers with $10 million or more in annual sales were required to update their labels by January 1, 2020; manufacturers with less than $10 million in annual food sales were required to ...
Food Additive Status List | FDA Aug 25, 2022 · May be added to medical food at levels not to exceed the amount necessary to meet the distinctive nutritional requirements of the disease or condition for which the food is formulated, and for ...
What is required on a food label? - USDA A meat and poultry label is required to contain 8 features. These are: the product name, inspection legend and est. number, handling statement, net weight statement, ingredients statement, address line, nutrition facts, and safe handling instructions. These requirements are found in the Code of Federal Regulations (9CFR 317.2/381 Subpart N).
Nutrition chap 12 quiz Flashcards | Quizlet The Nutrition Labeling and Education Act of 1990 requires which of the following? A. restaurants must provide nutrition details to consumers B. most foods be labeled using a standardized format C. all food be labeled with calorie amounts D. packaged food will include nutrient claims E. all health claims must be placed on the front of the packaging
PDF General Food Labeling Requirements - California required. Other label information, such as health claims and nutrient content claims, are voluntary. These label statements are based on the following statutes: 1. Fair Packaging and Labeling Act (FPLA) of 1967, 2. Federal Food, Drug, and Cosmetic Act (FD&C) 3. Nutrition Labeling and Education Act of 1990 (NLEA), 4.
Overview of Food Ingredients, Additives & Colors | FDA VerkkoA color additive is any dye, pigment or substance which when added or applied to a food, drug or cosmetic, or to the human body, is capable (alone or through reactions with other substances) of ...
Homework 2 Flashcards | Quizlet Eating the proper proportion of foods is referred to as: balance. You are reading a food label which indicates that the product contains 22% of the DV of calcium, 2% of the DV of Vitamin C and 30% of the DV from fat. Based on this information which of the following statements is correct? This product is high in calcium.
Guidance for Industry, Q7A Good Manufacturing Practice ... Sep 24, 2001 · I. INTRODUCTION (1) A. Objective (1.1) This document is intended to provide guidance regarding good manufacturing practice (GMP) for the manufacturing of active pharmaceutical ingredients (APIs ...
Food Ingredients & Packaging | FDA Irradiation of Food & Packaging FDA provides regulatory and scientific information about irradiated food and packaging. Irradiation may be used to increase shelf-life and reduce harmful...
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration You must calibrate: (1) Before first use; and. (2) At the frequency specified in writing by the manufacturer of the instrument and control; or. (3) At routine intervals or as otherwise necessary to ensure the accuracy and precision of the instrument and control. (c) You must repair or replace instruments or controls that cannot be adjusted to ...
FDA Food Packaging Guidelines for 2022 | Newprint Different Elements of Food Packaging Guidelines Explained 1. Common Name Common name ("ice cream", "sandwich") must be visibly displayed with the format of the food (if there are different formats available on the market). 2. Net Quantity Net quantity is the amount of food in the food packaging. It must be given in weight, measure, or count.
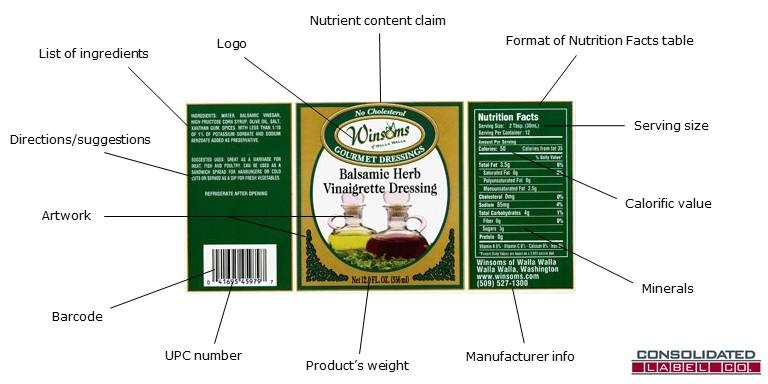
Nutrition- Chapter 2 Mastering health Flashcards | Quizlet Answer: A five components on the cereal box that identify the food packaging requirements mandated by the FDA. 1. Nutrition facts panel 2. Food name 3. net weight of food 4. ingredients 5. manufacturer name and address Not: Label claims food image trademark Nutrition facts panel
Packaging and Labeling - Food and Drug Administration a) Gang-printed labeling is a sheet of labeling that contains ¨ Different drug products, strengths, or net contents of same drug b) Gang-printed sheets are prohibited unless well differentiated ¨...
Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables)...
Cosmetics Labeling Guide | FDA - U.S. Food and Drug … VerkkoRegulations [21 CFR 701.10] published by the FDA require that the PDP be large enough to accommodate all required label information with clarity and conspicuousness.
eCFR :: 21 CFR Part 312 -- Investigational New Drug Application (a) This part contains procedures and requirements governing the use of investigational new drugs, including procedures and requirements for the submission to, and review by, the Food and Drug Administration of investigational new drug applications (IND's). An investigational new drug for which an IND is in effect in accordance with this part is exempt from the premarketing approval ...
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (iii) The following sample label illustrates the provisions in paragraphs (c) and (d) of this section, including paragraph (d)(10) of this section, which permits modifications for small packages: (iv) The following sample label illustrates the provisions in paragraphs (c) and (d) of this section for a drug product marketed with cosmetic claims:
Packaging and labelling guide for cannabis products - Canada.ca VerkkoNot required. Not required. Required for allergens only. Not required. Required. 132.18(1)(n) 132.19(1)(i) 132.22(3) Nutrition facts table: must be displayed in the format set out in the document entitled Directory of Nutrition Facts Table Formats for Edible Cannabis. See section 8.2.2 of this guide. Not required. Not required. Not required ...
510(K) Submissions for Piston Syringes Guidance A kit label alone may suffice for all components only if the label consolidates the required information typically found in labeling for each individual kit component when sold separately in final ...
Resource Grains - USDA Aug 25, 2022 · Please refer to the “Criteria for Determining Acceptable Grain Items” section for more information on grains requirements in each CN program.. At least eighty percent of the grains offered weekly in the NSLP (7 CFR 210.10(c)(2)(iv)(B)) and the SBP (7 CFR 220.8(c)(2)(iv)(B)) must be whole grain-rich, and the other grain items offered must be enriched.
Guidance for Industry: Food Security Preventive Measures ... If a food establishment operator suspects that any of his/her products that are regulated by the FDA have been subject to tampering, "counterfeiting," or other malicious, criminal, or terrorist ...
Chapter 5: Food Labels Flashcards | Quizlet This fact remains true whether or not the milk proclaims it. What are the eight common allergens that have to be listed on food labels? 1) milk 2) eggs 3) fish 4) shellfish 5) tree nuts (cashews, walnuts, almonds, etc.) 6) peanuts 7) wheat 8) soybeans What are the three main reasons that food labels are so important?
Nutrition Facts Labeling — FDA Reader The minimum requirement is listed below (must be listed in this order): Vitamin D, Calcium Iron Potassium When additional vitamins and minerals are listed, the following order should be preserved: Vitamins (listed in order) Vitamin A Vitamin C Vitamin D Vitamin E Vitamin K Thiamin Riboflavin Niacin Vitamin B6 Folate Vitamin B12 Biotin
DailyMed - XARELTO- rivaroxaban tablet, film coated XARELTO ... Verkko2.11.2022 · XARELTO for the treatment of DVT and/or PE was studied in EINSTEIN DVT [NCT00440193] and EINSTEIN PE [NCT00439777], multi-national, open-label, non-inferiority studies comparing XARELTO (at an initial dose of 15 mg twice daily with food for the first three weeks, followed by XARELTO 20 mg once daily with food) to …
Nutrition Labels 101: What's Required? What's Optional? The FDA requires nutrients that fall into one of these categories be listed on a nutrition label only when it's necessary to bolster or prove the label's food label or marketing claim. To best help consumers make informed decisions about their food choices, the FDA says all nutrition labels must include these 13 components.
Optional Nutrients On The Food Label - LabelCalc So if you are struggling with your nutrition facts label or just want to streamline the label creation process, an easy-to-use online label creation software that clearly denotes optional nutrients makes the task of creating your food label a breeze. LabelCalc offers FDA-compliant web-based nutritional analysis and label-making that is easy to ...
FDA Food Product Labeling & Packaging Requirements - ESHA Servings per container Mandatory nutrients (total calories, total fat, saturated fat, trans fat, cholesterol, sodium, total carbohydrate, dietary fiber, total sugars, added sugars, protein, vitamin D, calcium, iron, potassium) In a parenthetical directly after the name of the ingredient within the ingredient statement.

![Food Labeling 101 - FDA Regulations Guide [2022] - Artwork Flow](https://global-uploads.webflow.com/5f59aa263c234bb74025de57/5fa4f8a355c6935dd2dde09d_Inner-Images-1.jpg)






















:max_bytes(150000):strip_icc()/Food-Labels-GettyImages-1141997545-2000-f99fe1c283c448528ef4ef264146c4fa.jpg)










Komentar
Posting Komentar