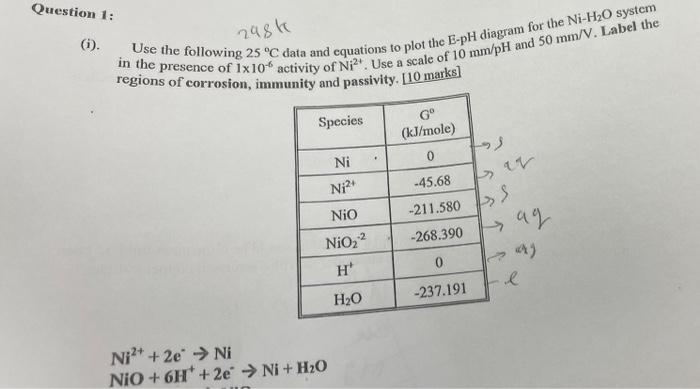
38 ph scale labeled diagram
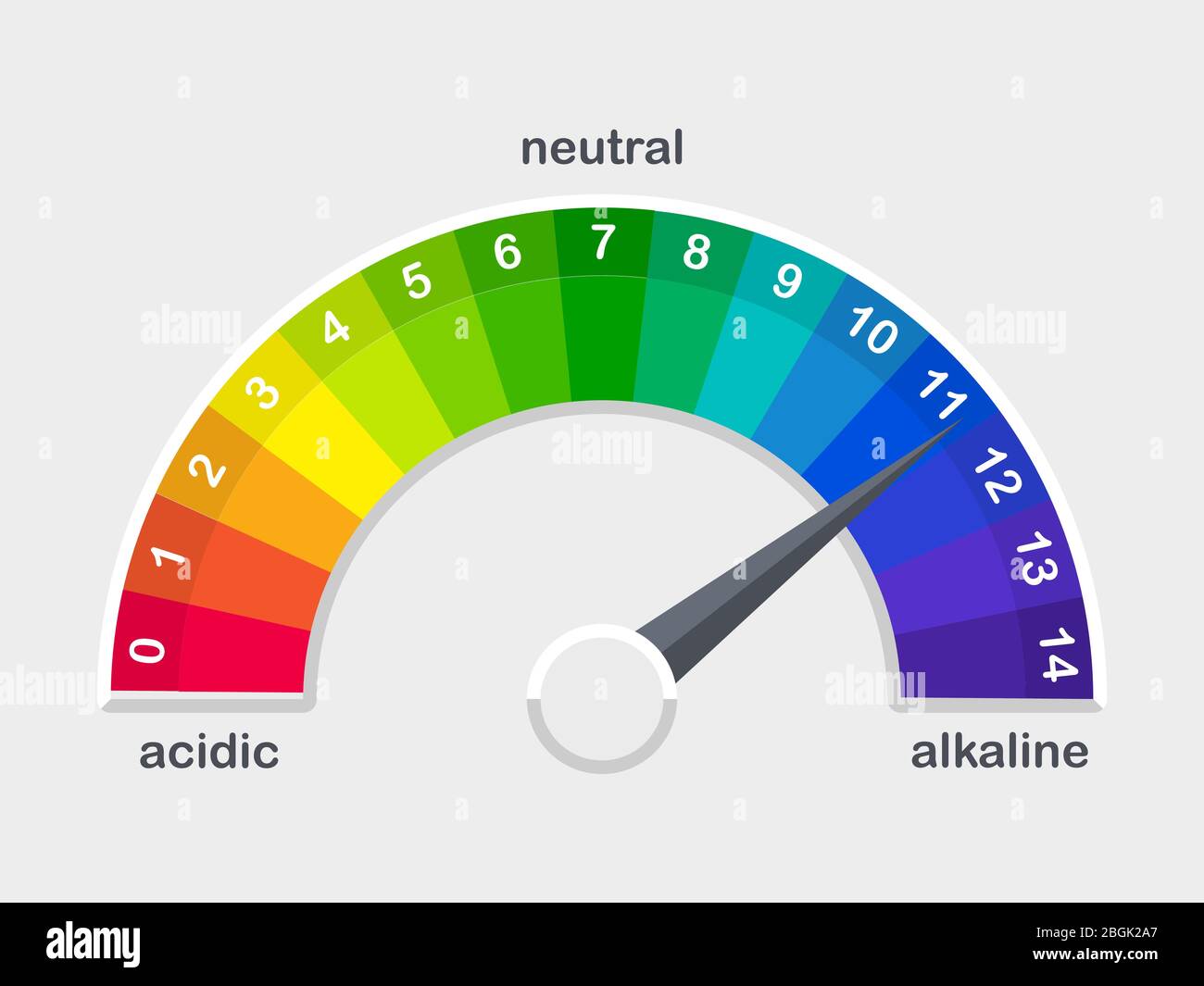
The pH scale Universal Indicator pH Color Chart diagram acidic... Activity: The Heart (for Kids). Do you know your heart? Label these heart parts. The pH scale - Acids, bases and salts - (CCEA) - BBC Bitesize The pH scale measures a solution's acidity or alkalinity. The range for the pH scale is 0 (strong acid) to 14 (strong alkali). pH 0 - 2: strong acid pH 3 - 6: weak acid pH 7: neutral pH 8 ...
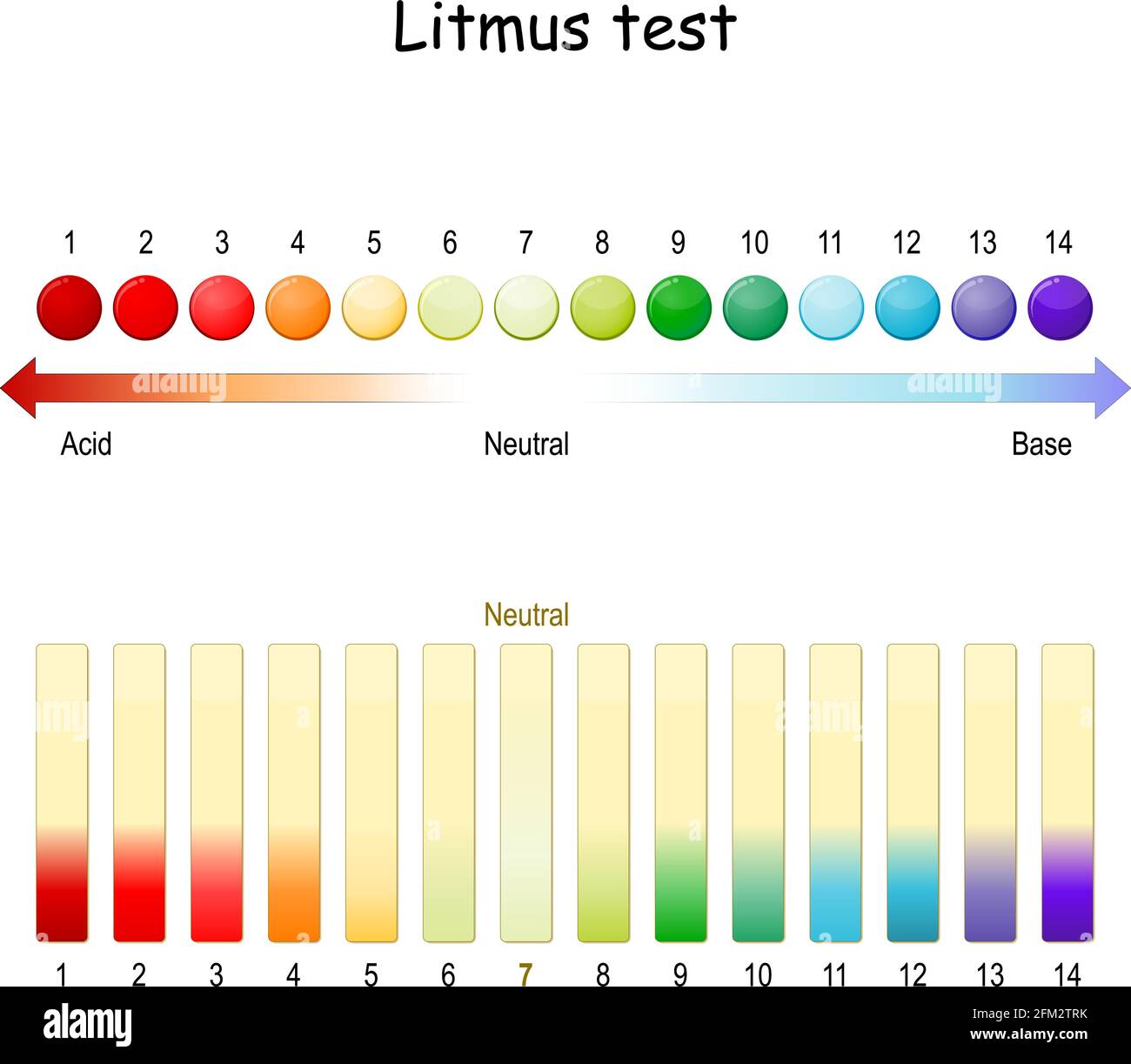
Chemistry: pH scale Diagram | Quizlet Neutral. pH of 7. Basic (Alkaline) A solution with a pH above 7. Greater concentration of hydroxide ions than concentration of hydrogen ions. pH scale according to the universal pH indicator strip. Red is acidic; green is neutral; blue is basic/alkaline. 1M hydrocloric acid (HCl) pH0.
Ph scale labeled diagram
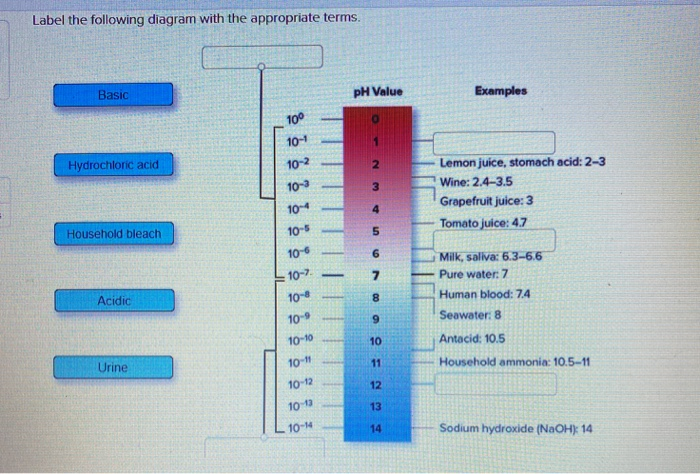
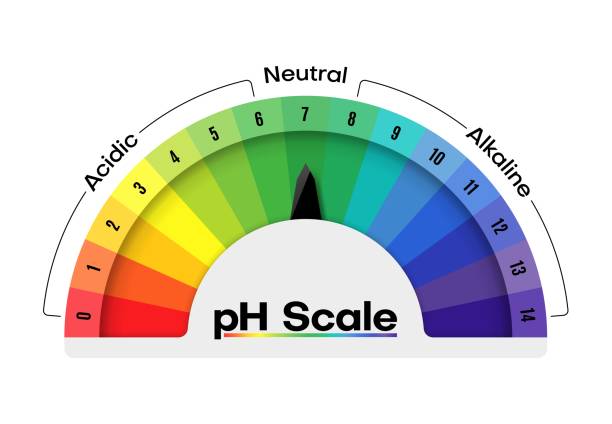
Review Quiz Ch. 2 Flashcards | Quizlet The pH scale ranges from 0 to 14 and is used to indicate the alkalinity of a solution. A solution with a (n) neutral pH has a (n) equal number of H⁺ and OH⁻ ions. In both directions on the scale, between the whole number value, there is a difference of ten times the concentration of hydrogen ions and hydroxide ions. Draw Neat and Labelled Diagram of pH Scale. - Shaalaa.com Draw Neat and Labelled Diagram of pH Scale. ... The range of ph values. Concept: Strength of Acidic or Basic Solutions. Report Error 6.3: The pH Scale - Mathematics LibreTexts Knowing the dependence of pH on [ \(\ce {H+}\) ], we can summarize as follows: If pH < 7, then the solution is acidic. If pH = 7, then the solution is neutral. If pH > 7, then the solution is basic. This is known as the pH scale. You can use pH to make a quick determination of whether a given water based solution is acidic, basic, or neutral.
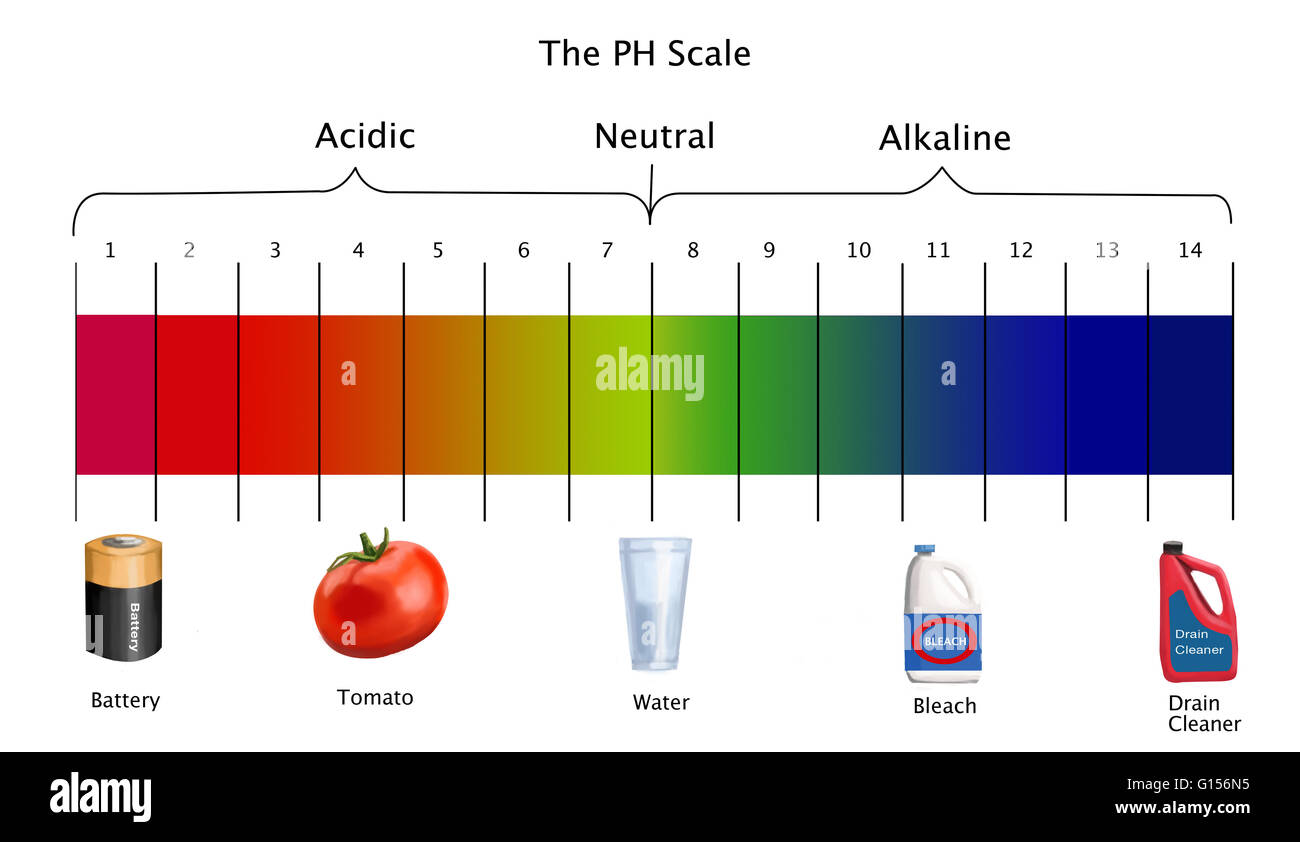
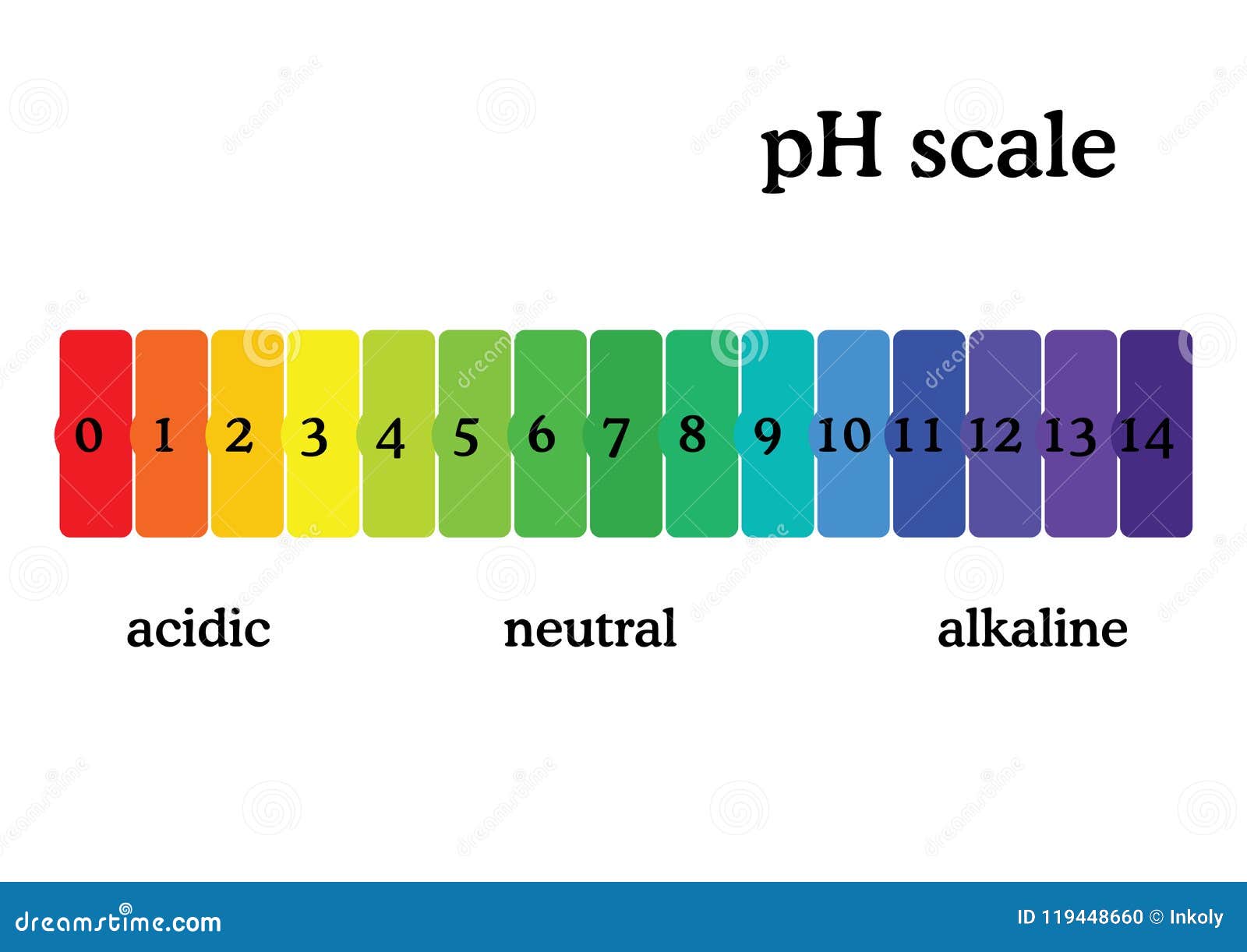
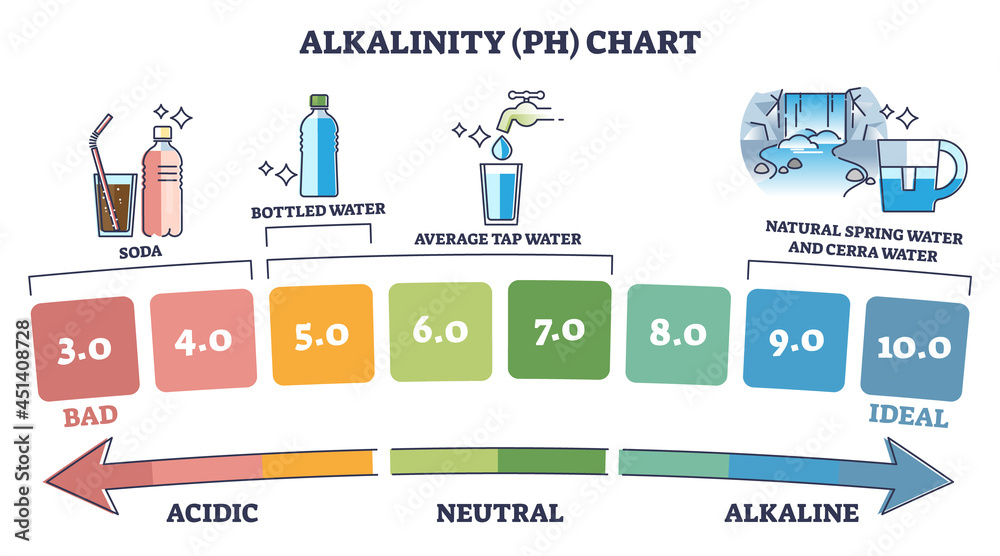
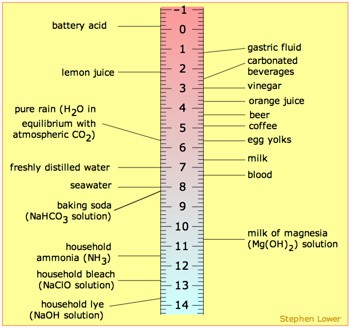
Ph scale labeled diagram. Diagram of the pH scale with examples of acidic, neutral and ... Download this stock image: Diagram of the pH scale with examples of acidic, neutral and alkaline substances. - G156N5 from Alamy's library of millions of ... Acid Rain Students Site: PH Scale - US EPA The scale has values ranging from zero (the most acidic) to 14 (the most basic). As you can see from the pH scale above, pure water has a pH value of 7. This value is considered neutral—neither acidic or basic. Normal, clean rain has a pH value of between 5.0 and 5.5, which is slightly acidic. However, when rain combines with sulfur dioxide ... Acids, Alkalis, and the pH Scale - Compound Interest Human blood has a pH value that's always slightly alkaline, between 7.35-7.45. If we were able to purposefully change the pH of blood outside of this small range, we could actually cause ourselves a good deal of harm; even a pH change of 0.5 either way could result in irreversible cell damage. pH Scale - Elmhurst University The pH scale measures how acidic or basic a substance is. The pH scale ranges from 0 to 14. A pH of 7 is neutral. A pH less than 7 is acidic. A pH greater than 7 is basic. The pH scale is logarithmic and as a result, each whole pH value below 7 is ten times more acidic than the next higher value.
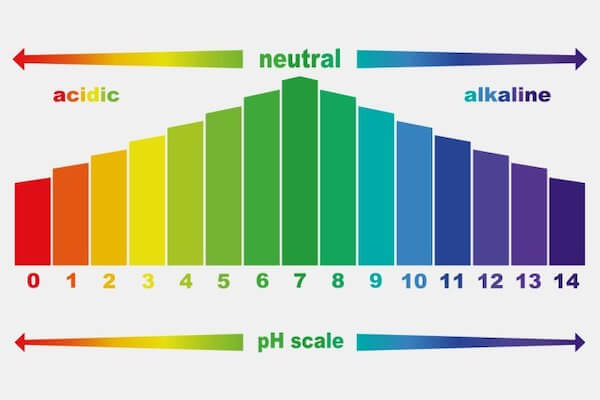
Question Video: Identifying the Correctly Labeled pH Scale Let's start by clearing some space on screen to take a closer look at a proper pH scale. The pH scale, with values generally falling between zero and 14, measures the acidity or basicity of a substance. Substances with a pH below seven are acidic. The lower the pH, the more acidic a substance is. The pH Scale | Biology for Non-Majors I | | Course Hero The pH scale, which measures from 0 to 14, provides an indication of just how acidic or basic a substance is. Most parts of our body (excluding things like stomach acid) measure around 7.2 and 7.6 on the pH scale (a 7 is neutral on the scale). If foreign strong substances dramatically change this pH, our bodies can no longer function properly. Draw neat and labeled diagram of pH scale? - Toppr Ask The neat and labeled diagram of pH scale is as shown. The range of pH is from 0 to 14. pH 7 corresponds to neutral pH. pH less than 7 corresponds to acidic pH. pH more than 7 corresponds to alkaline pH. Was this answer helpful? 0 0 Similar questions What colour does Universal Indicator go in a liquid with a pH of 9? Medium View solution > pH Scale | U.S. Geological Survey The pH scale measures how acidic an object is. Objects that are not very acidic are called basic. The scale has values ranging from zero (the most acidic) to 14 (the most basic). As you can see from the pH scale above, pure water has a pH value of 7. This value is considered neutral—neither acidic or basic.
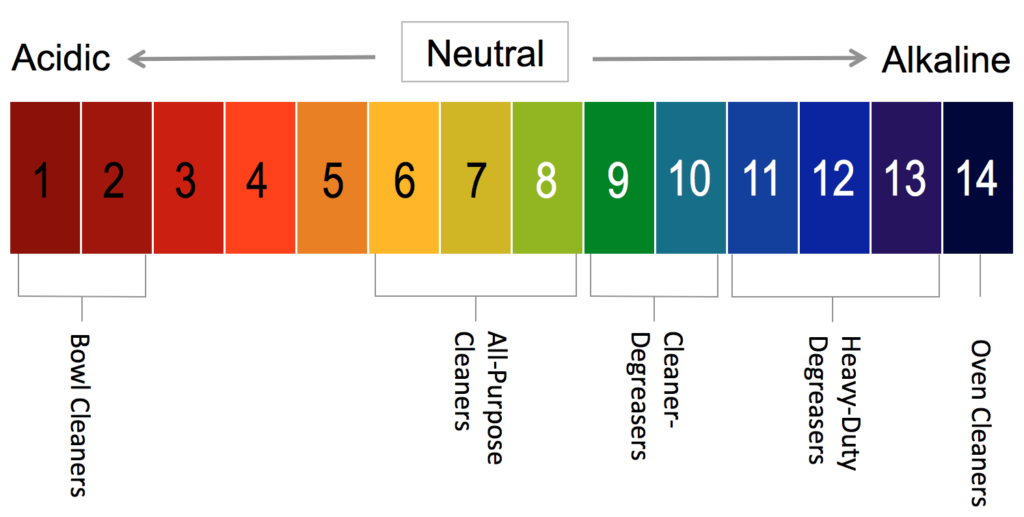
2,080 Ph Scale Images, Stock Photos & Vectors | Shutterstock pH scale diagram with corresponding acidic or alkaline values for common substances, food, household chemicals . Litmus paper color chart. Colorful flat vector illustration on white background. 1136369255 Using litmus paper to measure pH. 1509260183 Use soil PH meter for check the PH value 1714216936 ph scale vector graphic . acid to base pH Indicator Chart - Colors and Ranges - Science Notes and Projects A pH indicator or acid-base indicator is a chemical added in a small amount to a solution that causes a color change depending on the pH. This is a charge of common indicators, an explanation of how they work, and tips for choosing the right one for your needs. How to Use a pH Indicator pH - Wikipedia In chemistry, pH (/ p iː ˈ eɪ tʃ /), historically denoting "potential of hydrogen" (or "power of hydrogen"), is a scale used to specify the acidity or basicity of an aqueous solution.Acidic solutions (solutions with higher concentrations of H + ions) are measured to have lower pH values than basic or alkaline solutions.. The pH scale is logarithmic and inversely indicates the activity of ... The pH Scale | Biology for Majors I | | Course Hero The pH scale is, as previously mentioned, an inverse logarithm and ranges from 0 to 14 (Figure 1). Anything below 7.0 (ranging from 0.0 to 6.9) is acidic, and anything above 7.0 (from 7.1 to 14.0) is alkaline. Extremes in pH in either direction from 7.0 are usually considered inhospitable to life.
What is the pH scale and what does it measure? - BBC Bitesize The pH scale shows how acidic a substance is. It can be measured using a pH meter which gives a numerical value. The pH scale ranges from 0 (very acidic ) through 7 ( neutral ) to 14 (very...
The PH Scale - SlideShare The PH Scale. 1. •P- power •H- hydrogen • Thus we define pH as a negative logarithm of the molar concentration of hydrogen ions. • pH=-log [H+] 2. The pH scale, first proposed in 1909 by the Danish biochemist S.P.L. Sorensen, to describe the degree of acidity or basicity. 3.
Acids, Bases, & the pH Scale - Science Buddies The pH scale is theoretically open-ended but most pH values are in the range from 0 to 14. It's a lot easier to use a logarithmic scale instead of always having to write down all those zeros! By the way, notice how one hundred million million is a one with fourteen zeros after it? It is not coincidence, it is logarithms!
16.4: The pH Scale - Chemistry LibreTexts The pH scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. For example, a pH of 3 is ten times more acidic than a pH of 4. Likewise, a pH of 3 is one hundred times more acidic than a pH of 5. Similarly a pH of 11 is ten times more basic than a pH of 10.
pH Scale: Acids, bases, pH and buffers (article) | Khan Academy The pH scale is often said to range from 0 to 14, and most solutions do fall within this range, although it's possible to get a pH below 0 or above 14. Anything below 7.0 is acidic, and anything above 7.0 is alkaline, or basic. Image modified from " Water: Figure 7 ," by OpenStax College, Biology, CC BY 4.0. Modification of work by Edward Stevens.
pH Scale | U.S. Geological Survey Each number represents a 10-fold change in the acidity/basicness of the water. Water with a pH of five is ten times more acidic than water having a pH of six. As this diagram shows, pH ranges from 0 to 14, with 7 being neutral. pHs less than 7 are acidic while pHs greater than 7 are alkaline (basic). Learn more about pH Sources/Usage
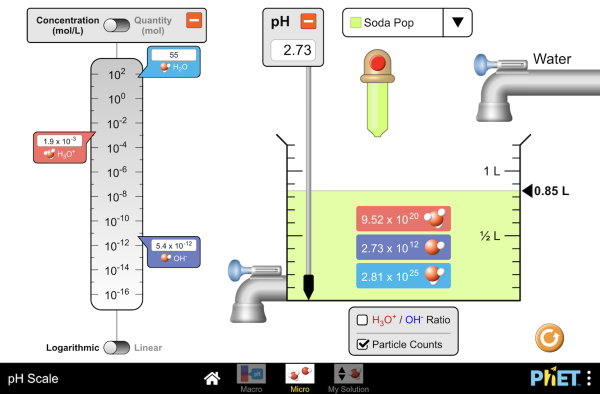
pH Scale - PhET pH Scale - PhET
The pH Scale - Chemistry LibreTexts The pH scale is logarithmic, meaning that an increase or decrease of an integer value changes the concentration by a tenfold. For example, a pH of 3 is ten times more acidic than a pH of 4. Likewise, a pH of 3 is one hundred times more acidic than a pH of 5. Similarly a pH of 11 is ten times more basic than a pH of 10. Properties of the pH Scale
The pH scale with some common examples - NOAA Pacific Marine ... Research. Ocean Acidification. Ocean Carbon Uptake. Ocean Carbon Storage. Coastal Carbon Dynamics. Observations. Volunteer Observing Ships (VOS) Buoys & Autonomous Systems. Hydrographic Cruises.
Draw a neat and labelled diagram of $pH$ scale. - Vedantu PH scale Answer Draw a neat and labelled diagram of p H scale. Answer Verified 249k + views Hint : We all are familiar with the term p H and p H scale. p H scale is a scale of acidity and basicity which tells about the acidic and alkaline behaviour of substance . Complete answer: > On the p H scale the value ranges from 0 to 14.
pH Calculator | How To Calculate pH? The pH scale (pH) is a numeric scale used to define how acidic or basic an aqueous solution is. It commonly ranges between 0 and 14 but can go beyond these values if sufficiently acidic/basic. The pH value is logarithmically and is inversely related to the concentration of hydrogen ions in a solution.
PH Scale in Simple Terms - YouTube The letters pH stand for the potential of hydrogen and is a measure of the concentration of hydrogen ions in a water-based substance? pH is organized into a scale of acids and bases with...
Why does the pH scale range from 0 to 14? Can it go ... - Science ABC The pH scale is used to determine whether a substance is acidic or basic, and to calculate how strong a chemical it is. A pH value is a number that ranges from 1 to 14 for most common chemicals, with seven being the middle or neutral point. Values below 7 are indicators of acidity, which increases as the number decreases, while values above 7 ...
pH | US EPA pH is an expression of hydrogen ion concentration in water. Specifically, pH is the negative logarithm of hydrogen ion (H +) concentration (mol/L) in an aqueous solution: pH = -log 10 (H +) The term is used to indicate basicity or acidity of a solution on a scale of 0 to 14, with pH 7 being neutral.
Ph Scale Illustrations, Royalty-Free Vector Graphics & Clip Art - iStock Vector pH scale. Acidic to normal to alkaline diagram. Rainbow laboratory paper indicator. Chemistry science concept. Balance measurement spectrum.
6.3: The pH Scale - Mathematics LibreTexts Knowing the dependence of pH on [ \(\ce {H+}\) ], we can summarize as follows: If pH < 7, then the solution is acidic. If pH = 7, then the solution is neutral. If pH > 7, then the solution is basic. This is known as the pH scale. You can use pH to make a quick determination of whether a given water based solution is acidic, basic, or neutral.
Draw Neat and Labelled Diagram of pH Scale. - Shaalaa.com Draw Neat and Labelled Diagram of pH Scale. ... The range of ph values. Concept: Strength of Acidic or Basic Solutions. Report Error
Review Quiz Ch. 2 Flashcards | Quizlet The pH scale ranges from 0 to 14 and is used to indicate the alkalinity of a solution. A solution with a (n) neutral pH has a (n) equal number of H⁺ and OH⁻ ions. In both directions on the scale, between the whole number value, there is a difference of ten times the concentration of hydrogen ions and hydroxide ions.

















Komentar
Posting Komentar